Researchers must manage numerous trial processes for various professional relationships, diverse cultures, backgrounds, and dialects worldwide. Language barriers for multilingual sites and teams can lead to misunderstandings or halt collaboration with peers, participants, and stakeholders.
When researchers can effectively communicate with all relevant parties, regardless of language or culture, they can produce better research and results.
With today’s technology and human expertise, your clinical trial translations don’t have to be expensive, full of errors, or complex. You can maintain team alignment and streamline your translation workflow using the right AI tools and native-speaking professional linguist services. This way, your research team can lead improved trials for better medical intervention and more innovative medical devices, vital drug development, and other pivotal research.
Read on to explore some common challenges that you face when translating clinical trials, see how more efficient processes work, and learn how to implement a translation plan that nets you faster, more accurate trial results through improved communication.
Why can clinical trial translation be so challenging?
Clinical trial translations are key for successfully communicating research, medical terminology, patient or participant interactions, and meeting regulations. Below are some translation challenges that medical professionals face:
1. Removing communication barriers
Culturally appropriate translations are not as simple as converting content word-for-word, even in a field so precise as medicine and science. Clinical trials involve multiple variables as scientists describe protocols, methodologies, results, and essential factors for both themselves and participants.
People from different backgrounds will interpret and perceive the message differently, whether it’s a term’s cultural value that influences the reader’s understanding, a historical-cultural background that influences the message’s meaning, or an idiom that doesn't translate well.
It’s essential to eliminate these communication barriers to ensure a successful clinical trial process and outcome. An AI-powered human approach like Smartling’s leverages machine translations’ speed and affordability and combines it with native, local expertise from humans who understand the culture.
2. Aligning with regulatory requirements
Clinical trial documents and communications require strict protocols. These documents also often require regulatory approval. Any error or misunderstanding can jeopardize or delay clinical research.
Translations should transform medical documents and communications for these regulatory bodies, not only to communicate the source text but also to establish and nurture relationships across site locations.
3. Communicating ethical standards and patient-centric language
Translations should fully communicate the risks, concerns, and benefits for participants who join a clinical research study. A lack of participant understanding for a specific detail or requirement poses ethical issues and compromises both the research and the participants. Patients and participants will understand translated materials based on educational, economic, and cultural backgrounds. Localized translations consider these differences to produce accurately translated text.
4. Maintaining consistency and accuracy
Have you ever read a piece of content written by more than one author? If it's a uniform piece, you might not even notice. But if the editors and authors fail to blend all the voices into one, the differences will stick out like a sore thumb.
In clinical trial translations, those experiences can cause frustration and confusion, which risks damaging regulatory, peer, and participant relationships.
While translations should always be accurate, they should also clearly offer a consistent tone of voice and vision. Organizations can implement linguistic validation to confirm that they receive credible and effective translations.
5. Translating technical and scientific content
Scientific language needs to be highly specific and precise.
The medical field’s terminology has many advantages, such as its many Latin-based and inspired terminologies, that can unite research communities worldwide. However, identifying key terms is insufficient for conducting a successful full-length trial.
Translations should be highly accurate and should thoroughly express ideas and protocols so that every researcher and participant understands the context, implications, and expectations.
6. Encouraging collaboration and peer communication
Clinical trial translations are also necessary for team and research collaboration. A lack of effective communication among multilingual researchers and stakeholders slows down their workflow.
For example, colors can influence a participant's perception of a clinical case. In the wrong context, the color white symbolizes mourning in China.
Researchers might know Spanish, French, Chinese, German, or another secondary language. But they often cannot translate the nuances and complexities that affect comprehension in different cultures and dialects.
Once you understand the challenges you face in clinical trial translations, you can start building a foundation that will make them possible.
How clinical trial translation services work
Clinical trial language services help scientists, patients, participants, and stakeholders bridge language barriers so they can efficiently perform impactful medical studies.
The most innovative translation solutions incorporate the following essentials:
- AI translation: Researchers can translate billions of words in seconds thanks to machine translation technology. Solutions like Smartling include translation memory and glossaries to implant local phrases and researcher voice styles in future translations for consistency.
- Machine quality control and assurance: Researchers can use Multidimensional Quality Metrics to ensure that medical translations meet the highest accuracy standards.
- Expert linguist verification, revision, and localization: With a translation management system and local linguists, translations undergo an efficient localization process to verify and revise translated content for each reader group.
These ingredients separate a robust translation workflow from inefficient content translation processes.
Use cases for clinical research translation services
Clinical trial translation services can support a wide range of use cases in your clinical trial:
1. Research and team communication for multi-site and multilingual settings
Translations should facilitate different types of interactions:
- Research collaboration to efficiently communicate processes and methodologies across sites
- Problem-solving, including developing treatments or finding ways to mitigate side effects
- Publications and documents, such as study reports, for global teams and stakeholders
- Relationships with regulatory bodies from different parts of the world or jurisdictions
Translations should clearly and accurately transform content for legal bodies.
2. Clinical trial agreements and legal forms
Participants and patients should understand the agreements and forms that researchers provide. This communication also includes direct clinical trial inputs like patient questionnaire forms or participant surveys.
3. Study protocols and results
Researchers depend on clear protocols and methodologies to perform accurate and credible studies.
When researchers analyze their findings, they must communicate them successfully to advance innovation and scientific research goals. Translations help with this communication, no matter the target language.
4. Participant and patient diary translations
Diary entries inform the study and its results, whether for medical devices, treatments, or new drugs—but understanding these documents can be more challenging in different languages and cultures.
Patients typically communicate in a language they're comfortable with. If you don’t understand that language, you’ll want to know that the translated version is accurate.
5. Event logs, case report forms, and back translations
You should translate any documentation and case information for relevant parties so they can accurately conduct the study.
One way to test a translation project is by performing back translations—translating clinical trial translations back into the source language. If the meaning is the same, you know you've done an excellent job. Translation services should pass a back translation test to confirm clinical trial quality. Governing bodies often request back translations, and researchers can provide them to successfully move forward with studies.
6. Patient recruitment
Clinical trial studies depend on great candidates for participation. To attract the right person, you can create localized patient information leaflets and marketing materials in a language that candidates understand and resonate with.
You can also translate onboarding materials, such as informed consent forms, information on adverse side effects, and other relevant details or documents, once participants join the study. Patients can then make a more informed decision throughout the trial process.
How to prioritize precision in clinical trial translation
How do you prioritize your approach with many different types of translations? It starts with understanding what makes up a localized translation.
The following are ways that you can produce translations with pin-point accuracy:
Accurate translations
Translations in the research and medical field should be precise. Whether you’re describing methodologies, protocols, patient details, and result summaries, every word can influence your study’s success.
For example, a researcher misunderstanding an integral part of a protocol could jeopardize the entire clinical trial. If participants don't fully understand the trial’s risks, they may become liable and cause ethical concerns.
You should translate everything you write and produce with complete accuracy.
Reader perceptions
Unfortunately, accuracy isn't as easy as adding one plus one. There isn't a single black-and-white answer. People are complex. Depending on their culture, country, dialect, and other essential factors, each group will perceive information differently.
A literal translation may not mean the same thing as your intended message. That's why it's essential to incorporate localized translations that make sure your documents communicate precisely what you want them to.
Localized translations play a pivotal role with diverse participants. For example, if a clinical trial focuses on participants with peanut allergies, localization should immediately start with the word “peanut.”
In Spanish alone, the correct translation depends on where your audience is located. "Cacahuate" is the word for peanuts in some of North and Central America. Spanish speakers in Spain vary slightly with "cacahuete.” And the Caribbean, Spanish-speaking communities in Florida, the rest of Central America, and much of South America use the word "maní" instead. 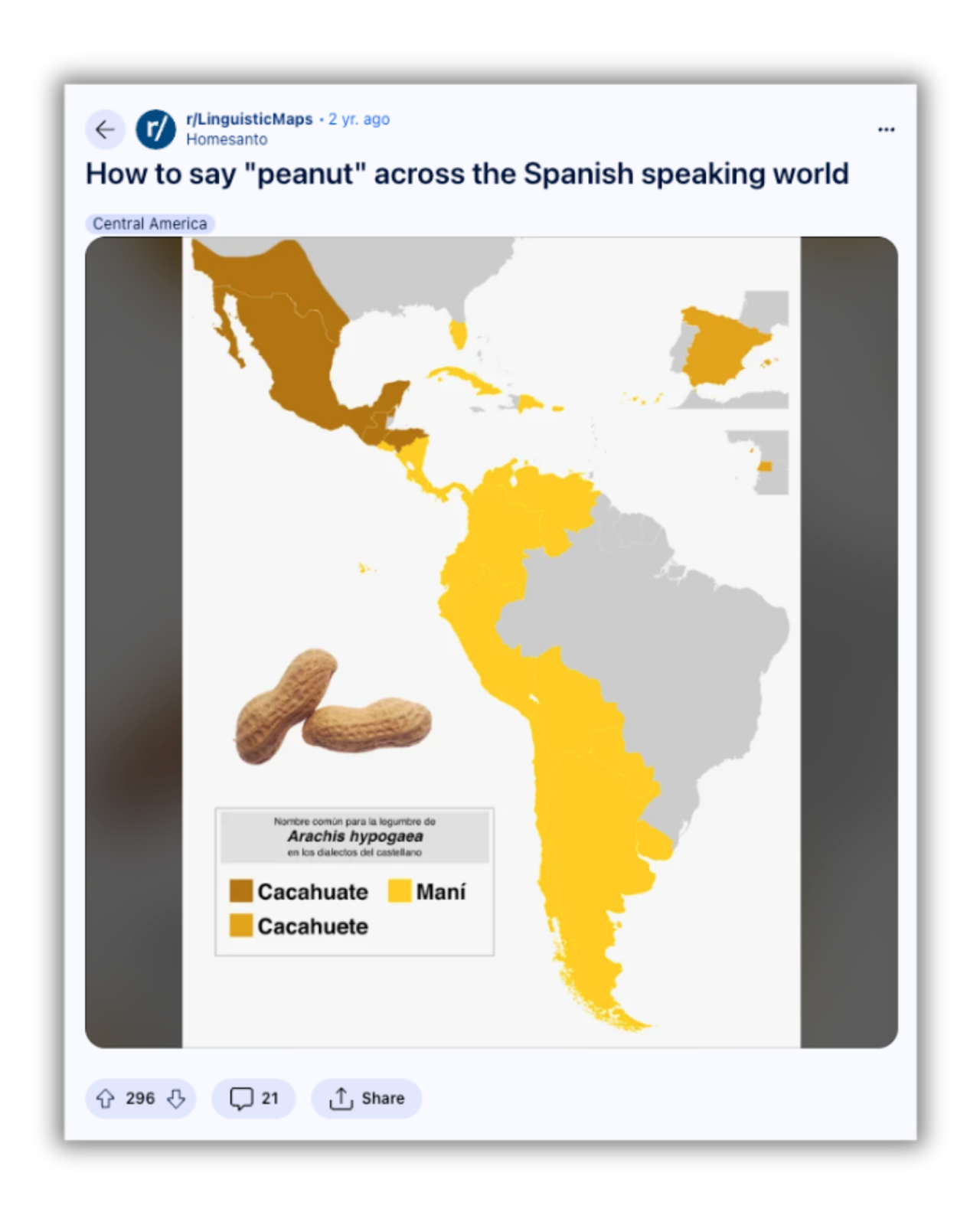 A visual representation of the different Spanish terms used for “peanut” worldwide (Source)
A visual representation of the different Spanish terms used for “peanut” worldwide (Source)
A healthy localization process involves the following:
- Finding specialized translators: Leverage native-speaking translators who know your local audience.
- Assessing quality and nuances constantly: Ensure that translations are always accurate and relevant by following shifting trends, meanings, and cultural impacts.
- Maintaining consistent terminology: Use consistent words, phrases, and brand voice to ensure consistency and avoid confusion.
The best way to incorporate these localization essentials is through a solution that offers the talent and tools to make successful translations possible, such as Smartling’s professional translation services and technologies.
Implementing translation services into existing clinical trial workflows
The translation process should be fast, simple, and effective. That’s why institutions and clinical research organizations should adopt tools and services that provide the most effective translation process. It’s critical that organizations and pharmaceutical companies choose the right solution to ensure clear, accurate translations.
Researchers can implement a solution by combining AI translations, management tools, and local expert translators into one workflow. Professional translation experts can then optimize the translation process using AI and powerful automation tools to collaborate with their team and edit content for local, accurately translated materials.
The criteria for choosing clinical trial translation services
When researchers have the green light and the funding to move forward, they need full focus, an aligned team, and clear communication. That’s why translation services are critical to the clinical trial research process.
Unfortunately, clinical trial translations can be slow, costly, and inaccurate.
You should look for three things when choosing a clinical trial translation service:
- Speed: Research translations should be as fast as possible to avoid delays and bottlenecks within the clinical trial workflow.
- Affordability: There's much to translate, and the process should be scalable while also staying within your budget.
- Accuracy: Translations should be correct and should convey the source text’s intended meaning.
Researchers once faced many setbacks, such as errors and long delays, in traditional translation settings where only human linguists translated content. Today, you can take advantage of the latest technologies as well.
You can affordably get fast and accurate translations through an AI-powered human approach.
For example, a translation service would use AI technologies, like Smartling’s Neural Machine Translation Hub, to translate billions of documents automatically and precisely. As linguists improve your tailored translation service, they can input specific phrases and words in a translation memory that is unique to your medical field, local participants, and other factors that influence how others perceive your message.
Once AI translates the content, experts and local linguists can verify translations and add any needed context for complex nuances for the culture or people group you are serving.
By leveraging the advantages of machine technology and human expertise, you can get clinical study translations that accurately communicate your messages and findings. This will allow you to more effectively publish research that can change lives and support your institution.
How Smartling powers world-class clinical trial translations
 Smartling is the all-in-one solution for clinical trial translation services.
Smartling is the all-in-one solution for clinical trial translation services.
Smartling is a powerful solution for clinical trial translations because of the powerful technology and services it offers researchers. When clinical trial teams adopt Smartling’s expert translation services, they get an AI-powered human translation solution.
You can use AI technologies, workflow management, and nuanced expert translation services to produce correct document translations the first time so everyone understands them. You also get continuous support from Smartling to successfully implement translations throughout the clinical trial.
You no longer need to deal with communication barriers that can jeopardize or slow down your clinical trial process. Instead, you can research confidently, speed up your process, and communicate medical findings that can change the world, save lives, and support your organization.
Book a meeting today to find out how Smartling can streamline your clinical trial communications to further your impact on medical advancements—no matter the language, culture, or dialect.

.jpg)





