The global pharmaceutical market has grown significantly in the past two decades, reaching about $1,607 billion in 2023. With this growth, the need for pharmaceutical translation has also increased, as brands set their eyes on diverse, multilingual audiences.
However, this expansion into new global markets also poses a huge challenge. Incorrect pharmaceutical translations can have very serious consequences in the medical field. From dosage instructions to side effect warnings, the margin for error is very low for pharmaceutical translations.
For companies that are looking to target markets across the globe, high-quality translations are not just a goal—they’re a necessity. But how do these companies achieve them?
In this article, we’ll look deeper at the role of accuracy in pharmaceutical translation and discover how pharmaceutical brands can achieve high-quality translations.
The critical role of translation in the pharmaceutical industry
Today, precise translations play a vital role at all levels of pharmaceutics, from groundbreaking clinical trials to your local pharmacy. To illustrate the widespread impact that translation has in the pharmaceutical industry, let's explore some key areas of influence:
Research and development documentation
The search for new treatments knows no borders. Because of this, translating research findings, clinical study data, and scientific papers is necessary to keep all involved parties in the loop.
Imagine, for example, that there is a breakthrough discovery in a German lab. Without swift, accurate translations, that knowledge might stay locked away, delaying potential treatments worldwide.
On the other hand, take the real-life example of COVID-19 vaccine development. Researchers worldwide shared data at remarkable speeds, bridging language gaps in the process. Rapid translations fueled this global teamwork and reduced vaccine development time from years to months.
Regulatory compliance
Getting a new drug approved is tricky, especially when you're dealing with different languages. Accurate translations play an important role in determining whether or not regulatory authorities will approve a new drug.
Authorities like the Food and Drug Administration and the European Medicines Agency have strict language standards. A small error in document translation during drug approval could lead to delays that might last for months. In severe cases, authorities may reject the application entirely.
Manufacturing processes and quality control
Consistency plays a crucial role in the pharmaceutical manufacturing process. Accurately translating standard operating procedures guarantees that companies manufacture drugs the same way every time, whether they’re located in Puerto Rico or Poland.
Pfizer, which has manufacturing sites worldwide and serves over 180 countries, relies heavily on translated documentation. The company’s approach to maintaining consistent quality worldwide shows the power of well-executed translations in upholding global manufacturing standards.
Product labeling and packaging
Have you ever had to squint at a medicine label to see what it says? Now, imagine trying to read it in a language you hardly know. Correctly translating product labels extends far beyond compliance—it can also directly impact patient safety and may save lives. However, each nation has its own regulations for labeling medication, which makes the translation process even more complicated.
The challenge lies in fitting vital information in multiple languages onto tiny packages. Pharmaceutical manufacturers must strike a delicate balance between comprehension and clarity using innovative packaging designs. 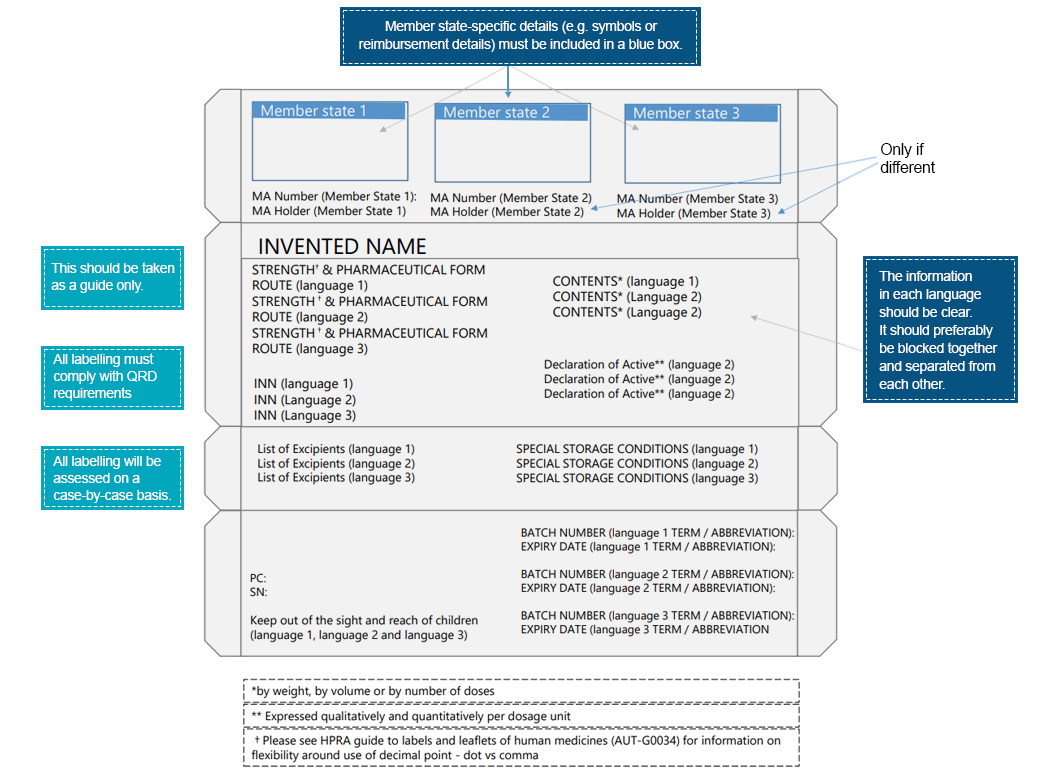 An example of multilingual pharmaceutical product labeling from the Health Products Regulatory Authority (Source)
An example of multilingual pharmaceutical product labeling from the Health Products Regulatory Authority (Source)
Marketing and patient education materials
Translated marketing content acts as a gateway for companies to access new markets. It's about more than promoting products—translations also help companies provide accurate information. When linguists translate and adapt information leaflets and other educational materials for other languages and cultures, they help patients understand how to properly and safely take medications.
Translations foster trust among pharmaceutical companies, healthcare providers, and patients. When a doctor in rural China can confidently prescribe a drug that Spanish researchers developed, that shows the power of effective pharmaceutical translation at work.
5 key strategies for ensuring pharmaceutical translation accuracy
Accurate translations in the pharmaceutical industry bridge language gaps while safeguarding patients’ lives. However, a small mistranslation can lead to potential health risks, rejected regulatory submissions, and damaged brand reputations.
Let's explore how top companies can ensure accuracy in their translations:
1. Always employ subject matter experts
Imagine that you're trying to explain quantum physics to a child. Tricky, right? That's what it's like when a translator without the correct subject matter expertise tackles a dense medical text. To overcome this challenge, pharmaceutical companies should work with translators who are well-versed in both the target language and medical terminology.
These companies should also collaborate with experienced medical translation providers or establish internal translation teams with relevant expertise. Doing so allows them to more effectively communicate complex medical concepts across different languages and minimizes the likelihood of harmful misunderstandings.
2. Implement robust terminology management
Proper terminology management is important for maintaining consistency across translations. This ensures that even the smallest errors, like “acetaminophen” suddenly becoming “paracetamol” halfway through a document, don’t slip through.
This process involves more than simply listing words. It also requires developing glossaries that provide context, usage notes, and approved translations for every term. Additionally, you can use a tool like Smartling's computer-assisted translation (CAT) tool to enhance terminology management. The CAT tool automatically suggests approved terms, flags inconsistencies, and maintains a centralized, up-to-date glossary across all your translation projects. This tool also seamlessly integrates with translation workflows, enabling access to real-time, active translation memory and pre-programmed quality checks.
3. Establish rigorous quality assurance
In the pharmaceutical industry, you can't just have a "good enough" translation. You need to conduct multiple stages of reviews, including back translation, to verify that critical documents communicate the same message in all languages.
You can use Multidimensional Quality Metrics (MQM) to assess and monitor translated content quality across several categories. Think of it as a report card for your translations. With MQM, you can catch errors, identify patterns of errors, and use analytics to make more strategic decisions. For example, if your metrics show a spike in terminology errors, it might be time to revisit and update your company's glossary. Or if stylistic issues keep popping up, maybe your brand’s style guide needs a refresh.
To achieve these results, many companies turn to advanced solutions that help cut down on manual work while maintaining translation quality. Smartling's AI-Powered Human Translation (AIHT) service, for instance, delivers an impressive MQM score of 98 so you receive the highest-quality translations every time.
4. Embrace cultural adaptation
Even perfectly translated content might not be effective if it doesn't resonate with your target audience.
Cultural adaptation in pharmaceutical translation involves more than just word-for-word translations. It also requires understanding local health beliefs, medical practices, and even color associations. To effectively reach your target audience, it's important to collaborate with language experts who understand these cultural nuances and can craft content that truly connects with your audience.
5. Leverage a translation management system
Maintaining accurate translations across multiple languages and markets is no small feat. To make this possible, you’ll need the right tech stack. Enter the translation management system (TMS), a comprehensive platform that streamlines the translation process and enhances overall efficiency.
Smartling's TMS provides more than just basic translation tools. Its integrated translation memory helps you maintain consistency across all your content, while automated quality checks catch potential errors before they become problems. When combined with Smartling's AIHT service, which integrates machine learning with human expertise, the result is elevated accuracy and a simplified translation process.
Maintaining consistency across pharmaceutical translation workflows
Another important aspect of pharmaceutical translation is consistency. Maintaining consistency across languages requires a combination of technological tools, human expertise, and strategic processes. Let's look at four key methods that pharmaceutical companies can use to maintain consistency in their translations:
- Translation memory: This tool stores translated words and phrases (strings) in each language for reuse in other content where the same content appears. Translation memory not only speeds up the translation process but also guarantees that key terms and phrases remain consistent across all documents.
- Glossaries and style guides: These assets help translators navigate the nuances of your brand’s voice and terminology. An up-to-date glossary acts as a dictionary of approved terms, while a comprehensive style guide outlines your brand’s tone and style preferences.
- TMS: This platform brings together translators, reviewers, and internal teams to improve real-time communication and share resources. This collaborative approach helps translation teams catch inconsistencies early and keeps everyone on the same page.
- Integrations: Integrating your TMS with systems like Salesforce and Marketo preserves consistency between your source content and translations across various marketing and sales channels. This seamless flow of information helps you maintain a unified brand voice, regardless of the platform or language.
Unlock pharmaceutical translation excellence with Smartling
While accurate and consistent pharmaceutical translations do eliminate language barriers, they’re also critical to patient safety, regulatory compliance, and global business success. With so much at stake, choosing a translation partner with industry-specific expertise and advanced technology is crucial for pharmaceutical companies that want to succeed in international markets.
This is where Smartling comes into play. Smartling offers a comprehensive suite of tools, services, and workflows that meet the unique challenges of pharmaceutical translations. These include:
- A cutting-edge TMS designed to centralize and streamline your translation workflows
- Tools like translation memory and Smartling's linguistic asset management service, which maintaining consistency and reduce costs
- Integrations with popular systems like Salesforce and Marketo for enhanced efficiency
- Specialized pharmaceutical translators who are proficient with intricate medical terminology and regulatory standards
- Strict quality assurance processes that ensure accuracy and compliance
Ready to elevate your pharmaceutical translations? Explore how Smartling can transform your global communication strategy—book a meeting today.
%20052925%20-%20AI%20Translation%20101%20(1).png)
.jpg)